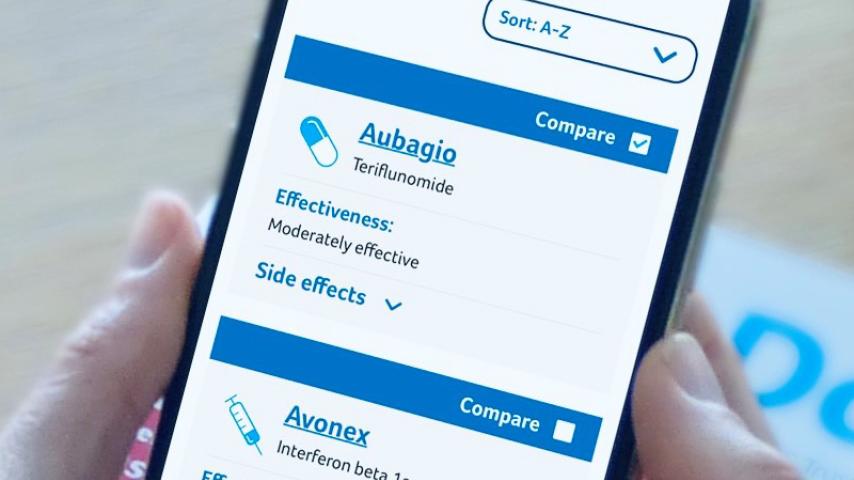
Tecfidera (dimethyl fumarate) is a disease modifying drug (DMD) for relapsing remitting MS.
You take Tecfidera as a pill twice a day to reduce the number and severity of relapses. It reduces the number of relapses by about one half (50%).
Common side effects include flushing and gastric upset (feeling sick, diarrhoea and stomach pains).
What is Tecfidera used for in MS?
Tecfidera is a disease modifying drug (DMD) for relapsing remitting MS. You have fewer relapses than you might have had with no treatment and any relapses you do have should be less severe.
Tecfidera is a more effective (category 1.2) DMD; in clinical trials people taking Tecfidera had about 50% fewer relapses than people taking placebo. In clinical trials, MRI scans showed that people taking Tecfidera had fewer, smaller or no new areas of active MS (lesions). Tecfidera may also slow down the build-up of disability associated with MS.
In England, Wales and Northern Ireland, the drug can be prescribed for adults with active relapsing remitting MS. In Scotland, Tecfidera is approved for adults with relapsing remitting MS.
Tecfidera has been approved for use on the NHS since 2014. It can only be prescribed by a neurologist.
Tecfidera is not recommended during pregnancy.
If you are trying for a family, talk to your MS nurse or neurologist about whether you should continue to take Tecfidera until you are pregnant.
If you become pregnant while on Tecfidera, your neurologist or MS nurse may recommend you stop taking it.
You take Tecfidera as a pill, twice daily with food.
To give your body a chance to get used to the drug and reduce the impact of side effects, you start on a low dose for the first week, increasing to the full dose in the second week.
What side effects could I get with Tecfidera?
Common side effects include:
- flushing and feeling hot
- gastrointestinal upset - diarrhoea, feeling sick, stomach pains
You are more likely to have these side effects when you first start taking Tecfidera (mostly during the first month). Most people have mild to moderate side effects which tend to go away over time.
A neurologist or MS nurse may suggest ways to reduce these side effects including:
- Reducing the dose temporarily, returning to full dose within one month
- Taking aspirin before each dose to prevent flushing (longterm use of aspirin is not recommended)
- Taking doses on a full stomach to reduce gastrointestinal upset – experience suggests this needs to be a balanced meal rather than a light snack.
Common side effects (affecting more than 1 person in 100)
- flushing and feeling hot
- gastrointestinal upset (feeling sick, diarrhoea, abdominal pain, vomiting, indigestion)
- decrease in white blood cells
- rash
- increased levels of liver enzymes
- ketones and protein in urine
Cases of progressive multifocal leukoencephalopathy (PML) have been reported for people taking Tecfidera. The risk of developing PML on Tecfidera is considered very low but if you are worried, discuss your concerns with your MS team.
When you start taking Tecfidera, you should be informed of the early signs and symptoms of PML. These can be similar to an MS relapse, so it is important to report any new or worsening symptoms. If PML is suspected during treatment, an MRI scan should be performed and Tecfidera suspended until PML has been excluded. The cases of PML have occurred in people who have had very low levels of lymphocytes (a type of white blood cell) for a long period of time. Once you've started treatment you should have blood tests every three months to monitor your blood cell counts.
Further advice has been published for health professionals concerning monitoring for PML.
A full list of side effects is included in the manufacturer's Patient Information Leaflet.
Assessment before treatment
Before starting Tecfidera, you should have blood and urine tests to measure blood cell counts and to check liver and kidney function. It is also important that you have had a recent (within 3 months) MRI scan.
Assessment during treatment
Once you've started treatment you'll have blood tests every 3 months and urine tests at 3 months, 6 months and then every 6 to 12 months thereafter. These tests monitor your blood cell counts and liver and kidney function. Depending on local practice, you may be able to have the tests at your GP surgery or you may need to attend a hospital clinic.
During the coronavirus outbreak, you may find that your regular blood and urine tests happen less frequently, may take place in a different location or may temporarily stop. The Association of British Neurologists has assessed the risks and benefits of blood monitoring for people taking DMDs, and has recommended a safe minimum schedule during this period. The recommendation for Tecfidera is that your blood tests can take place every six months, so long as your white blood cell levels remain over 0.5. If you notice any new or worsening MS symptoms, you should contact your MS team.
The way Tecfidera works is not fully understood, but laboratory studies suggest that it may work in two ways:
- reduces the inflammation caused when the immune system attacks myelin, resulting in less damage to myelin
- protects nerve cells from damage caused by chemicals released during the immune attack
Evidence for the effectiveness of Tecfidera has come from two large clinical trials.
- DEFINE - Tecfidera compared to placebo (2012)
This two year study compared Tecfidera taken either two or three times daily and placebo in more than 1,200 participants with relapsing remitting MS. Compared to placebo, Tecfidera twice daily reduced the number of relapses in one year by 53%. Tecfidera twice daily reduced the risk of 3 month disability progression by 38%.
- CONFIRM - Tecfidera or Copaxone compared to placebo (2012)
This two year study with 1,232 participants was similar to DEFINE, but with an additional group who took Copaxone (glatiramer acetate) for comparison.
Tecfidera reduced the number of relapses in one year by 44% for the twice-daily dose compared to placebo. In contrast, Copaxone reduced the number of relapses by 29% compared to placebo. The reduction in disability progression observed in the DEFINE study was not seen in the CONFIRM study.
-
National Institute for Health and Care Excellence (NICE)
Dimethyl fumarate for treating relapsing-remitting multiple sclerosis
NICE Technology Appraisal Guidance 320
Full guideline (link is external)
Gold R, et al.
Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis.
New England Journal of Medicine 2012;367:1098-107.
Summary (link is external)
Fox RJ, et al.
Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis.
New England Journal of Medicine 2012;367:1087-97.
Summary (link is external)