Disease modifying drugs (DMDs) are a group of treatments for people with multiple sclerosis. Most DMDs are for people with relapsing remitting MS (RRMS), but there are some that are licenced for use by people with progressive MS. For people with RRMS, disease modifying drugs reduce the number of relapses you might experience as well as reducing the severity of any relapses you do have.
There is a wide range of drugs approved for use by the NHS in the UK. Each drug offers a different combination of benefits and risks.
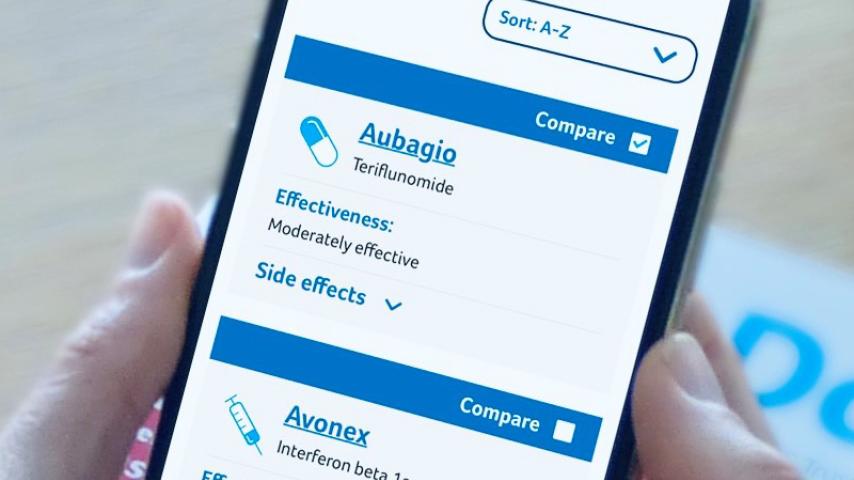
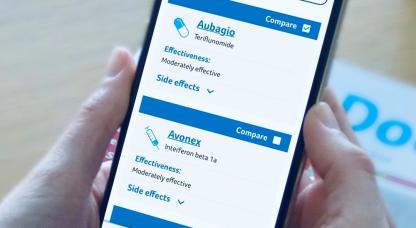
Our MS Decisions guide is here to help you find out more about the DMDs for RRMS, explore your options, and discuss starting or switching between one of the drugs with your MS team.
Other treatments are available:
Which DMDs are approved for use in the UK?
The following disease modifying drugs have been approved for use by the NHS in the UK for people with MS. The availability of each drug may vary in England, Scotland, Wales and Northern Ireland. See individual drug pages for more information on availability.
If you have primary progressive MS (PPMS) or secondary progressive MS (SPMS), you may be eligible to take a disease modifying drug under certain conditions. We have more information about Ocrevus for PPMS and Mayzent for SPMS.
What are the benefits of DMDs?
The main benefits of taking one of the DMDs are:
- Fewer relapses
- Less severe relapses
- Reduce the build-up of disability which can occur if you don't recover completely from relapses
DMDs work with different parts of the immune system to reduce the inflammation caused by MS to nerve cells in the brain and spinal cord. This helps reduce the number and severity of relapses.
Inflammation does not always result in a relapse or visible symptoms. This silent activity may mean that although you are feeling well, there may still be changes caused by your MS that can only be seen on a brain scan. MRI scans show that taking a DMD can lead to fewer, smaller or no new areas of damage (lesions) in the brain and spinal cord. Treating the visibly active (relapses) as well as the silently active aspects of MS is a new goal that is emerging in MS treatment. This goal is often called no evidence of disease activity (NEDA). The aim is to reach a point where you are free of visible (relapses) and invisible (changes seen only on brain scans) MS disease activity.
On average, people with relapsing remitting MS have one or two relapses a year. In the NHS, DMDs are approved for prescription according to how frequently you have been having relapses. The NHS describes relapsing remitting MS as being either active or very active:
Active relapsing remitting MS
Your MS may be described as active if you've had two relapses in the previous two years.
Very active relapsing remitting MS
Your MS may be described as very active if it has been either:
- highly active despite treatment - if you continue to have relapses even though you've been taking a DMD for a year
OR
- rapidly evolving severe - if you have had two or more severe, or disabling relapses in the previous year and show areas of new damage (lesions) on two consecutive MRI scans.
Some research suggests that DMDs work best when they are started as soon as possible after diagnosis, effectively before there is any sign of disability, to reduce the build-up of damage to nerve cells.
It may take three to six months from starting treatment for a DMD to become fully effective, so the benefits of treatment may not be immediately obvious.
Most people will continue to have a background of symptoms. DMDs are not able to repair nerve damage already caused by MS so they cannot reverse existing symptoms.
When used to treat clinically isolated syndrome, some of the DMDs have been shown to delay further episodes that would lead to a definite diagnosis of MS.
Although most DMDs are prescribed for people with relapsing remitting MS, some of the DMDs can be prescribed for people with secondary progressive MS (SPMS) or primary progressive MS (PPMS).
Do DMDs reduce long term disability?
For most DMDs, clinical trials are conducted over 2-4 years, which means that the evidence on long term disability and disease progression is limited at the time when the drug is licensed. After licensing, longer term data on safety and efficacy is collected, analysed and reported.
For the injectable DMDs (Avonex, Betaferon, Copaxone and Rebif), this data comes from the UK Department of Health Risk Sharing Scheme. This study followed 5,000 people taking one of the injectable DMDs over 10 years, and compared them to an untreated historical group.
The researchers found that the treated group developed lower levels of disability compared to the untreated group, and that the DMDs did slow the progress of the disease. However, the treatment effect wanes over the longer term. This implies that a person gets more long term benefit from a DMD if they start treatment when they are relatively younger and less affected by disability.
In 2019, research showed that people who received early treatment with a highly effective DMD (Tysabri or Lemtrada), did better over five years than those who started on a less effective DMD. Even though the group who started on a less effective DMD were closely monitored and switched to a highly effective DMD if their MS remained active, they still experienced worsening disability and a faster increase in their EDSS than those who started on a highly effective DMD at the outset.
What are the risks of taking DMDs?
All the DMDs can potentially cause side effects - see information on individual drugs for more details. Some people do not experience any side effects at all, some find they ease after the first month or two as their body adapts to the drug and others may find that side effects persist. Your MS team will give you advice on how to reduce the impact of side effects. A few people find side effects cause problems or are intolerable and have to change or stop treatment.
Some of the DMDs are associated with less common but potentially serious and life-changing side effects. There are mechanisms in place to minimise the risks for people taking one of these drugs, such as advice on warning signs, additional tests and regular check-ups. Your MS team will step in quickly if there is any cause for concern. However, some people may feel that, despite regular monitoring, they are unwilling to take the risk of developing serious side effects. Others may feel that the benefits of the drugs outweigh the risks.
How do we know the DMDs work?
Phase III clinical trials provide the main evidence for the effectiveness of DMDs.
DMD clinical trials recruit hundreds of people and last for about two years. The main aim of these trials is to compare the number of relapses in people taking the new drug with those taking the standard treatment or placebo. Trials can include other measures such as the number of lesions seen on MRI scans and changes in disability which last for 3 months or more. Any side effects the participants report are recorded and monitored.
Comparing one drug with another
Relatively few clinical trials have directly compared one DMD with another.
Comparing the results of one clinical trial with another may not give an accurate picture of which DMD is more effective than another. One of the main problems is that the groups of people recruited for each of the trials will be different, in terms of average age, gender mix, where they live and how long they have been diagnosed with MS. For example, the earliest large-scale DMD trials were carried out 25 years ago; it's likely that, on average, participants in the early studies will have had MS for a longer time than those recruited for more recent clinical trials.
Statistical techniques have been developed which take account of differences in the groups of participants in trials; this approach have been used to compare results from separate clinical trials and provide an indirect comparison of how effective the drugs are at reducing relapse rates.
For MS Decisions we have grouped the DMDs according to how effectively they reduce relapse rates, based on broad categories recommended in guidelines published by the Association of British Neurologists (ABN):
- Category 1.1: moderately effective - reduces relapses by one third (30%)
- Category 1.2: more effective - reduces relapses by one half (50%)
- Category 2.0: highly effective - reduces relapses by two thirds (70%)
How are DMDs approved for use by the NHS?
Before a DMD is approved for use by the NHS it will have been thoroughly investigated in clinical trials to establish its effectiveness and safety.
NICE (National Institute for Health and Care Excellence) appraises new medicines for England and Wales. They look at the evidence on how well a new DMD works, any drawbacks or limitations the drug may have and the cost effectiveness of treatment. In Scotland, appraisal is carried out by SMC (Scottish Medicines Consortium). NICE guidance is reviewed and adapted for use in Northern Ireland.
Other organizations may also influence a drug's availability within the NHS. To guide prescribing, NHS England has published a commissioning policy and a treatment algorithm for the DMDs and requires neurologists to enter details of each prescription into Blueteq, an online system for managing high cost drugs. The AWMSG (All Wales Medicines Strategy Group) may appraise a new drug if NICE is not expected to carry out an assessment within the next twelve months. The ABN (Association of British Neurologists) has published guidelines for prescribing DMDs in the UK.
-
Palace J, et al.
Effectiveness and cost-effectiveness of interferon beta and glatiramer acetate in the UK Multiple Sclerosis Risk Sharing Scheme at 6 years: a clinical cohort study with natural history comparator.
Lancet Neurology 2015;14(5):497-505.
Summary (link is external)
Scolding N, et al.
Association of British Neurologists: revised (2015) guidelines for prescribing disease-modifying treatments in multiple sclerosis.
Practical Neurology 2015;15(4):273-279.
Full article (link is external)
NHS England
Clinical commissioning policy: disease modifying therapies for patients with multiple sclerosis (MS) May 2014
NHS England/ D04/P/b
Full policy (link is external)
Harding, K et al.
Clinical Outcomes of Escalation vs Early Intensive Disease-Modifying Therapy in Patients With Multiple Sclerosis
JAMA Neurol. Published online February 18, 2019. doi:10.1001/jamaneurol.2018.4905
Summary (link is external)